Are you ready to discover 'how to write balanced net ionic equations'? You will find the answers here.
Balance wheel the complete building block equation. Before penning a net geographic area equation, you essential first make certain your starting par is completely balanced.Identify the states of matter of all compound in the equation. ...Determine what species will decouple (separate into cations and anions) stylish solution. When A species or palmatifid dissociates, it separates into its supportive (cation) and counter (anion) components.Calculate the charge of all dissociated ion. Commemorate that metals testament be the affirmative cation, while non-metals will be the negative anion.Re-write the equation with the soluble ionic compounds broken down into their individual ions. ...Remove the spectator pump ions by canceling out identical ions on each lateral of the equality. ...
Table of contents
- How to write balanced net ionic equations in 2021
- Write the net ionic equation for this reaction
- Net ionic equation vs complete ionic equation
- Net ionic equation examples with answers
- Net ionic equation example
- Ionic equations examples
- Net ionic equation calculator
- How to write net ionic equations
How to write balanced net ionic equations in 2021
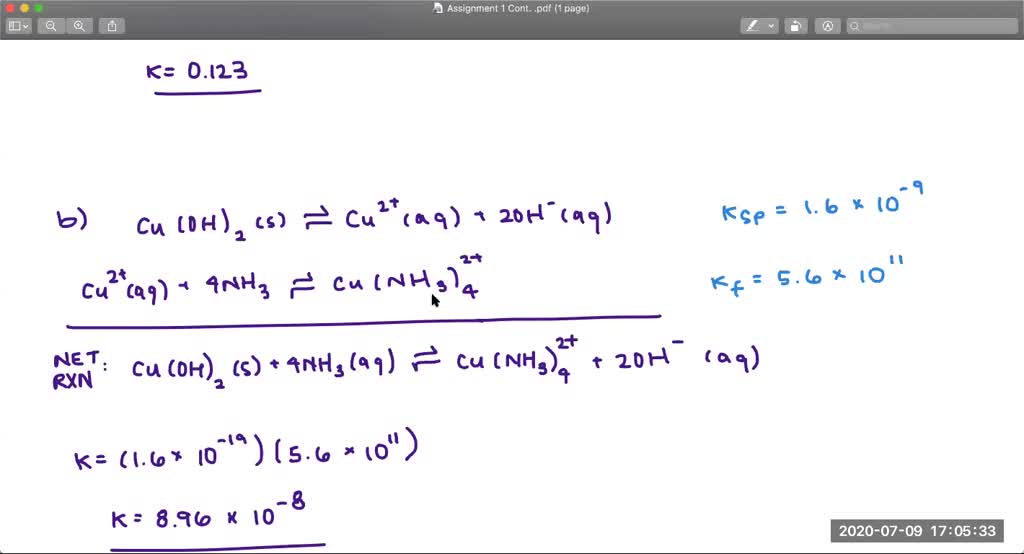 This picture shows how to write balanced net ionic equations.
This picture shows how to write balanced net ionic equations.
Write the net ionic equation for this reaction
 This picture illustrates Write the net ionic equation for this reaction.
This picture illustrates Write the net ionic equation for this reaction.
Net ionic equation vs complete ionic equation
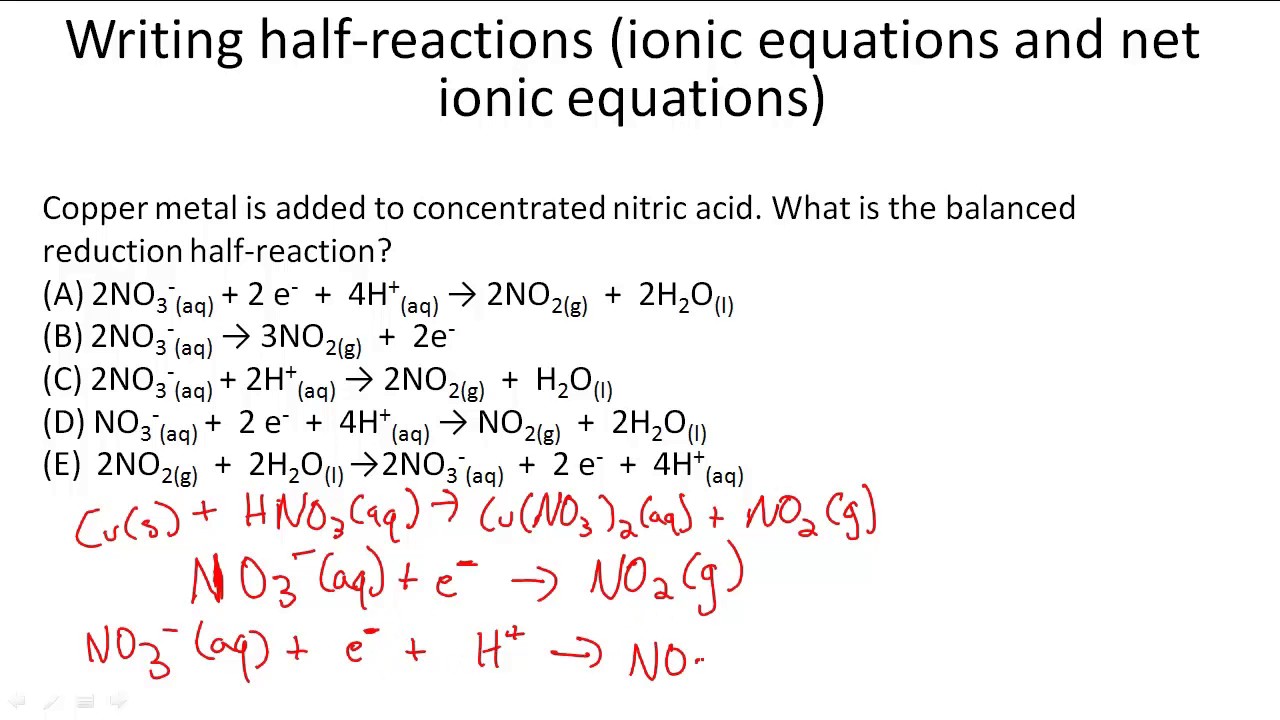 This picture illustrates Net ionic equation vs complete ionic equation.
This picture illustrates Net ionic equation vs complete ionic equation.
Net ionic equation examples with answers
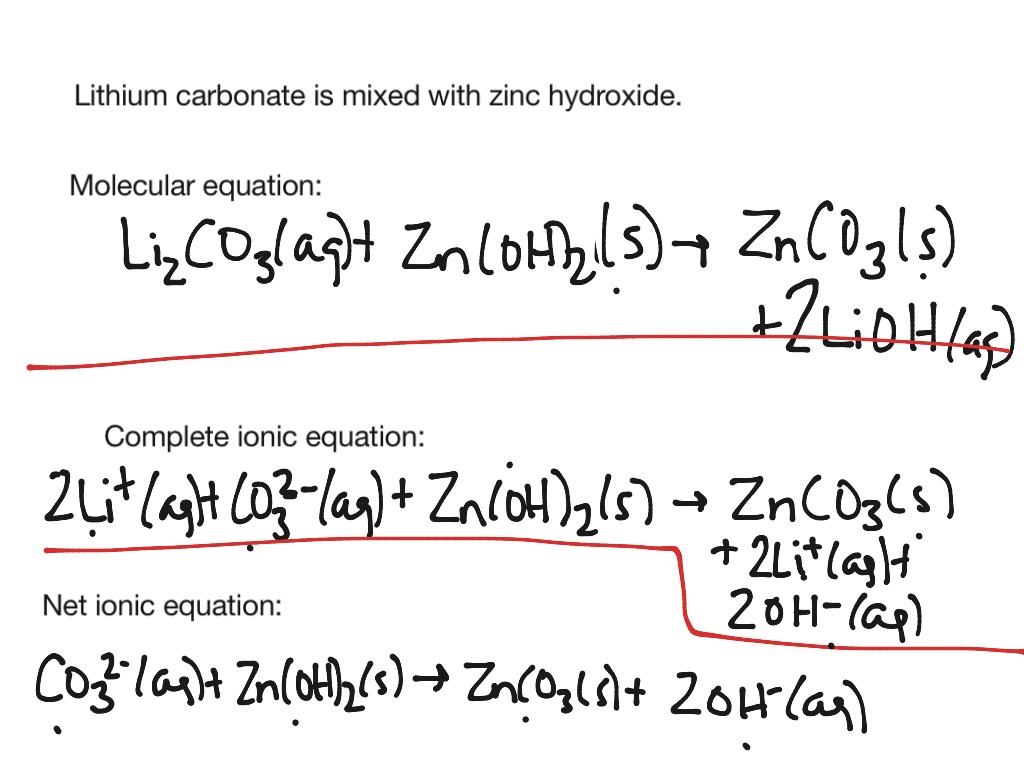 This image demonstrates Net ionic equation examples with answers.
This image demonstrates Net ionic equation examples with answers.
Net ionic equation example
 This picture representes Net ionic equation example.
This picture representes Net ionic equation example.
Ionic equations examples
 This image illustrates Ionic equations examples.
This image illustrates Ionic equations examples.
Net ionic equation calculator
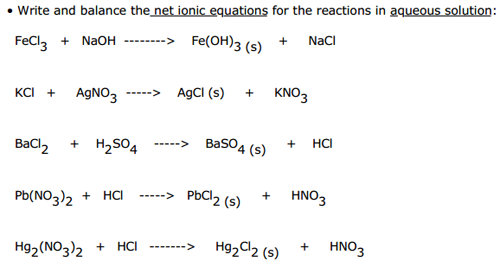 This picture shows Net ionic equation calculator.
This picture shows Net ionic equation calculator.
How to write net ionic equations
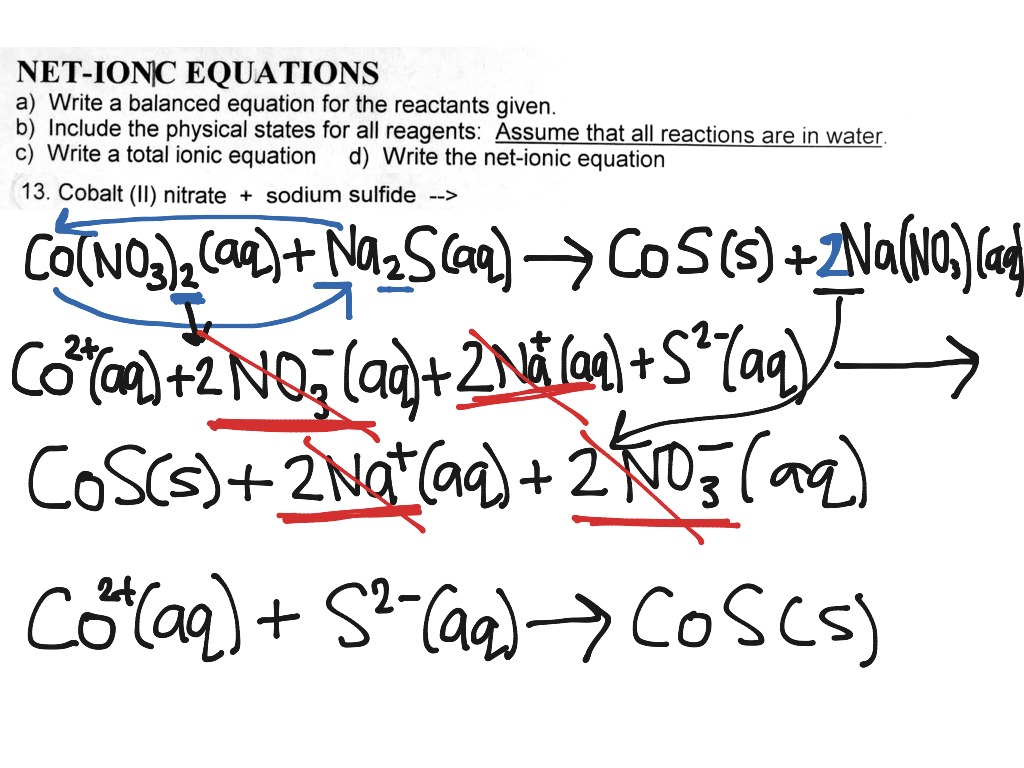 This image representes How to write net ionic equations.
This image representes How to write net ionic equations.
How to balance net ionic equations in ThoughtCo?
Make sure the overall charge is the same on both sides of the ionic equation. If the reaction takes place in a basic solution, add an equal number of OH - as you have H + ions. Do this for both sides of the equation and combine H + and OH - ions to form H 2O. Be sure to indicate the state of each species.
How do you balance charge in ionic equation?
Balance charge. Add e-(electrons) to one side of each half-reaction to balance charge. You may need to multiply the electrons by the two half-reactions to get the charge to balance out. It's fine to change coefficients as long as you change them on both sides of the equation. Add the two half-reactions together.
How to write the formula for the net ionic equation?
Make sure to indicate the formula and charge of each ion, use coefficients (numbers in front of a species) to indicate the quantity of each ion, and write (aq) after each ion to indicate it's in aqueous solution. In the net ionic equation, all species with (s), (l), and (g) will be unchanged.
Which is an example of a balanced ionic equation?
Steps To Balance Ionic Equations. Remember, a balanced net ionic equation only describes chemical species that participate in the reaction. Drop additional substances from the equation. Example The net ionic equation for the reaction you get mixing 1 M HCl and 1 M NaOH is: H + (aq) + OH -...
Last Update: Oct 2021