Are you desperately looking for 'williamsons ether synthesis'? You can find your answers here.
Table of contents
- Williamsons ether synthesis in 2021
- Williamson ether synthesis procedure
- Williamson ether synthesis base
- Williamson synthesis class 12
- Williamson ether synthesis reagents
- Williamson ether synthesis practice problems
- Williamson ether synthesis lab report
- Williamson synthesis is used to prepare
Williamsons ether synthesis in 2021
 This picture demonstrates williamsons ether synthesis.
This picture demonstrates williamsons ether synthesis.
Williamson ether synthesis procedure
 This image demonstrates Williamson ether synthesis procedure.
This image demonstrates Williamson ether synthesis procedure.
Williamson ether synthesis base
 This picture demonstrates Williamson ether synthesis base.
This picture demonstrates Williamson ether synthesis base.
Williamson synthesis class 12
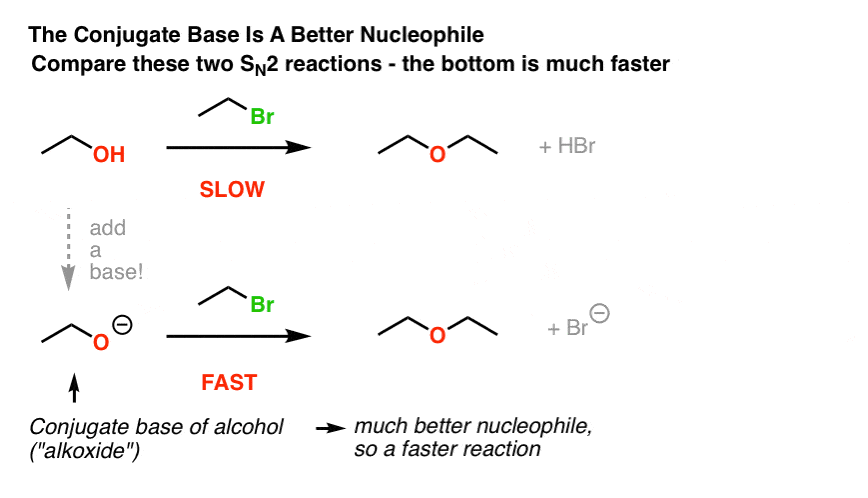 This image demonstrates Williamson synthesis class 12.
This image demonstrates Williamson synthesis class 12.
Williamson ether synthesis reagents
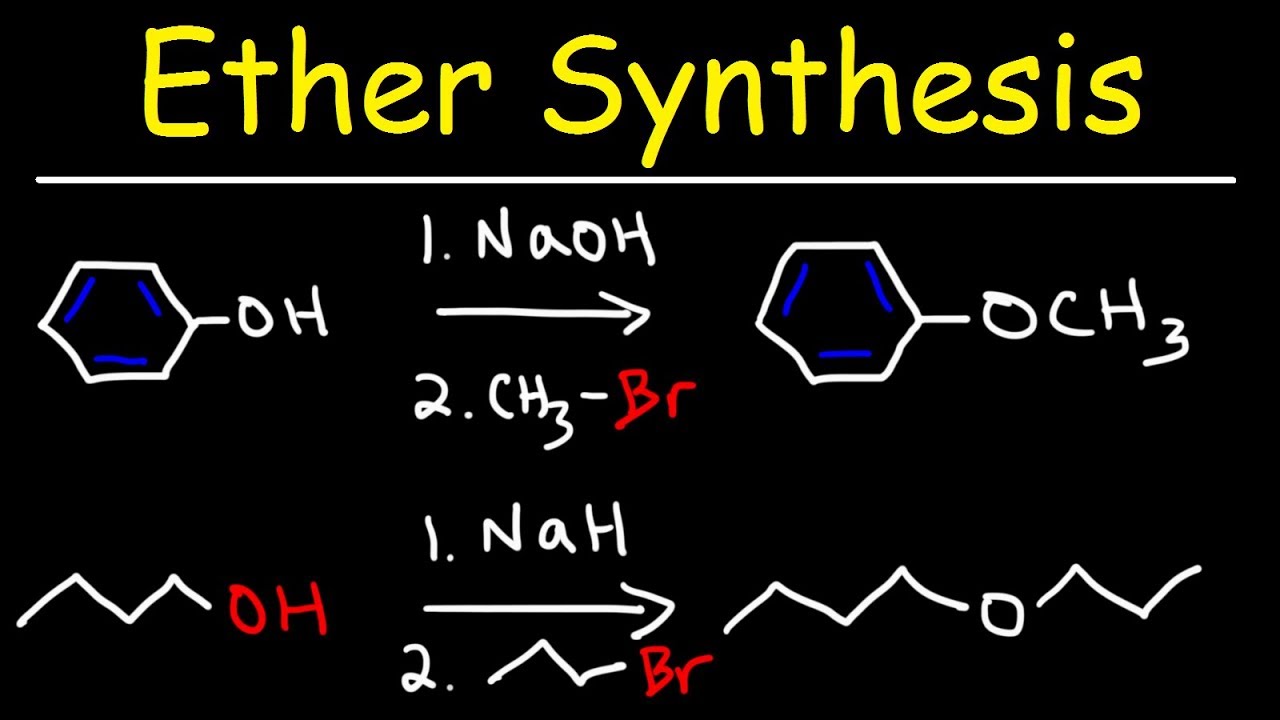 This image illustrates Williamson ether synthesis reagents.
This image illustrates Williamson ether synthesis reagents.
Williamson ether synthesis practice problems
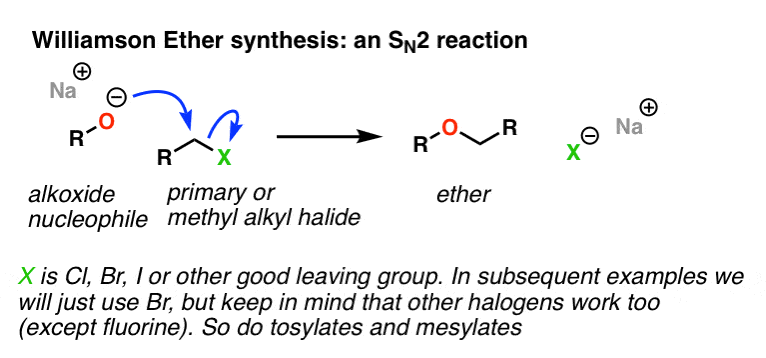 This image demonstrates Williamson ether synthesis practice problems.
This image demonstrates Williamson ether synthesis practice problems.
Williamson ether synthesis lab report
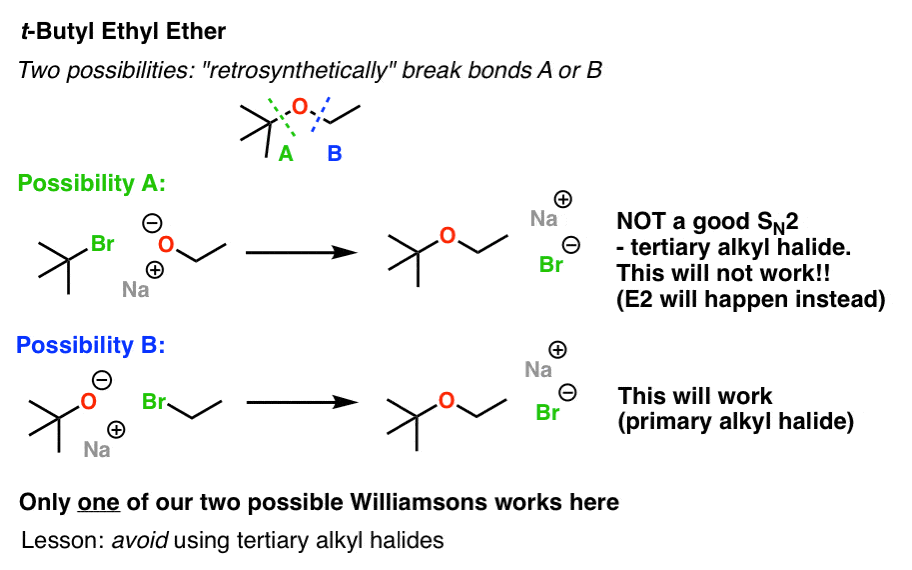 This image representes Williamson ether synthesis lab report.
This image representes Williamson ether synthesis lab report.
Williamson synthesis is used to prepare
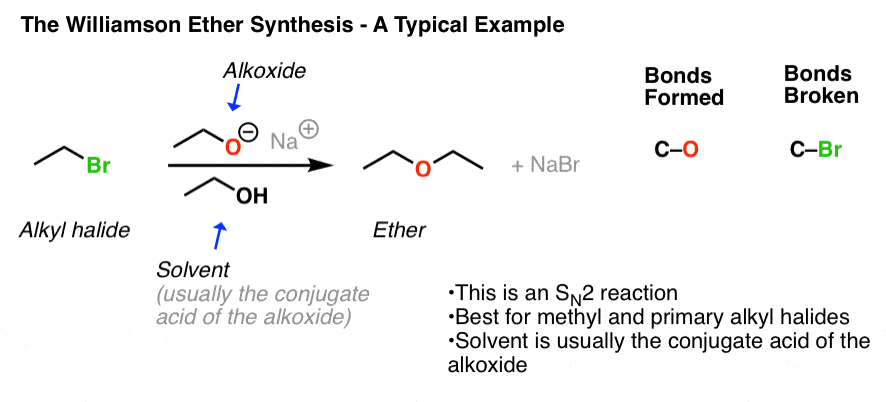 This picture demonstrates Williamson synthesis is used to prepare.
This picture demonstrates Williamson synthesis is used to prepare.
What kind of acid is used to make Williamson ether?
Ether synthesis by reaction of salicyaldehyde with chloroacetic acid and sodium hydroxide. The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide).
Which is a good candidate for Williamson synthesis?
Besides the alkyl halides, tosylates and mesylates are other excellent candidates for reacting with alkoxides in Williamson synthesis: Williamson Synthesis for Symmetrical and Unsymmetrical Ethers Williamson synthesis can be used to prepare symmetrical and unsymmetrical ethers:
How are alkyl halides used in Williamson's synthesis?
Action of alcoholic sodium alkoxide on alkyl halides to form ethers is known as Williamson’s synthesis. This reaction is given by primary alkyl halides.Tertiary alkyl halides preffer to undergo elimination. In this mechanism ,alkoxde ion attacks on carbon atom of alkyl halides by following SN² mechanism to form ether.
What kind of reaction is Williamson ether synthesis?
Williamson Ether Synthesis is a reaction that uses deprotonated alcohol and an organohalide to form an ether. Williamson Ether Synthesis usually takes place as an SN2 reaction of a primary alkyl halide with an alkoxide ion.
Last Update: Oct 2021